· Biomedical Disposable
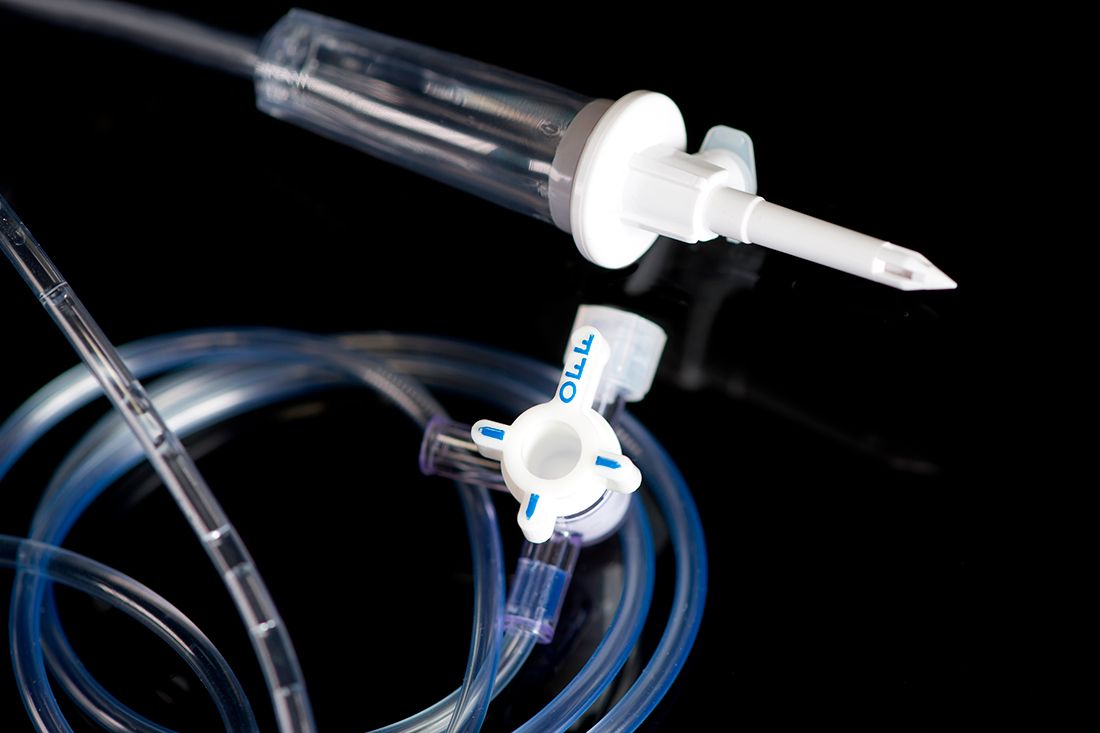
In the medical environment, reliability is a basic requirement. This means that guaranteeing the best possible functional and performance specifications of disposable devices is fundamentally important for manufacturing companies.
Medical devices producers must respect additional requirements, normally not necessary for other industries. These requirements normally concern health and safety.
The majority of medical and disposable devices are subject to various preliminary approval requirements. The leak and flow tests are requested and performed on 100% of the medical components and sets, since they enable non-conformities to be detected, faster, cheaper and more easily.
This check plays an essential role in guaranteeing the correct quality standards when manufacturing medical products.